Rare metal is an obstacle to using hydrogen as fuel for the green transition
When water is split, it can become an easily accessible source of hydrogen. However, there is a need for the process to proceed as efficiently as possible, and for as long as possible. Iridium helps with this, but since it is also a rare element, a new research project will look at whether it is possible to use less of it.

Science has known about the possibility of splitting water into oxygen and hydrogen for more than 200 years. In modern times, water as a source of hydrogen, can become important as part of the green transition, where hydrogen can play a role in transforming the chemical industry into a sustainable industry.
»Especially if you use renewable energy for it. Then you have electricity from renewable sources and water to produce hydrogen, and it can then be used to produce a lot of other chemicals. For example, sustainable fuels or fertilser,« explains Rebecca Pittkowski, assistant professor at the Department of Chemistry at University of Copenhagen.
However, there are various reasons why the splitting of water into oxygen and hydrogen does not yet play a major role in the green transition. One of them is that the process requires a catalyst, and the best known is iridium oxide. But iridium is also one of the rarest elements on the planet. Can we find a way to use less of it?
That is what Rebecca Pittkowski plans to investigate. She has received a Sapere Aude: DFF-Starting Grant from Independent Research Fund Denmark to support her work.
Taking an X-ray of the process
Iridium's key role is that the metal ensures stability in the process. So, if you use less iridium, the catalyst is no longer robust enough. This means that the process uses more and more electrical power and that the hydrogen production cannot be guaranteed over a sufficiently long period of time.
Therefore, Rebecca Pittkowski will literally take an X-ray image of the process. So, as the process progresses, it will be irradiated with high-energy X-rays to provide a nuanced picture of what is going on at a microscopic and atomic level.
Here, she and the rest of the project group, which will consist of two PhD students, will work with the center for electron acceleration that Lund University in Sweden has at its disposal, named MAX IV.
Insights may also be used for other types of catalysis
All in all, the project will extend over four years.
»I hope that by then we will have found a catalyst in which we use much less iridium than is needed right now. It can be a major contribution to the green transition,« says Rebecca Pittkowski, adding:
»I would also like to understand the processes that lead us to have less efficiency through the use of the catalyst, so that the splitting of water becomes less efficient. That is, a real in-depth understanding of the stability or instability of catalyst materials. Then you can also look at some other reactions than just water splitting and see how we can combine the different X-ray characterization methods and use it to understand catalysis.«
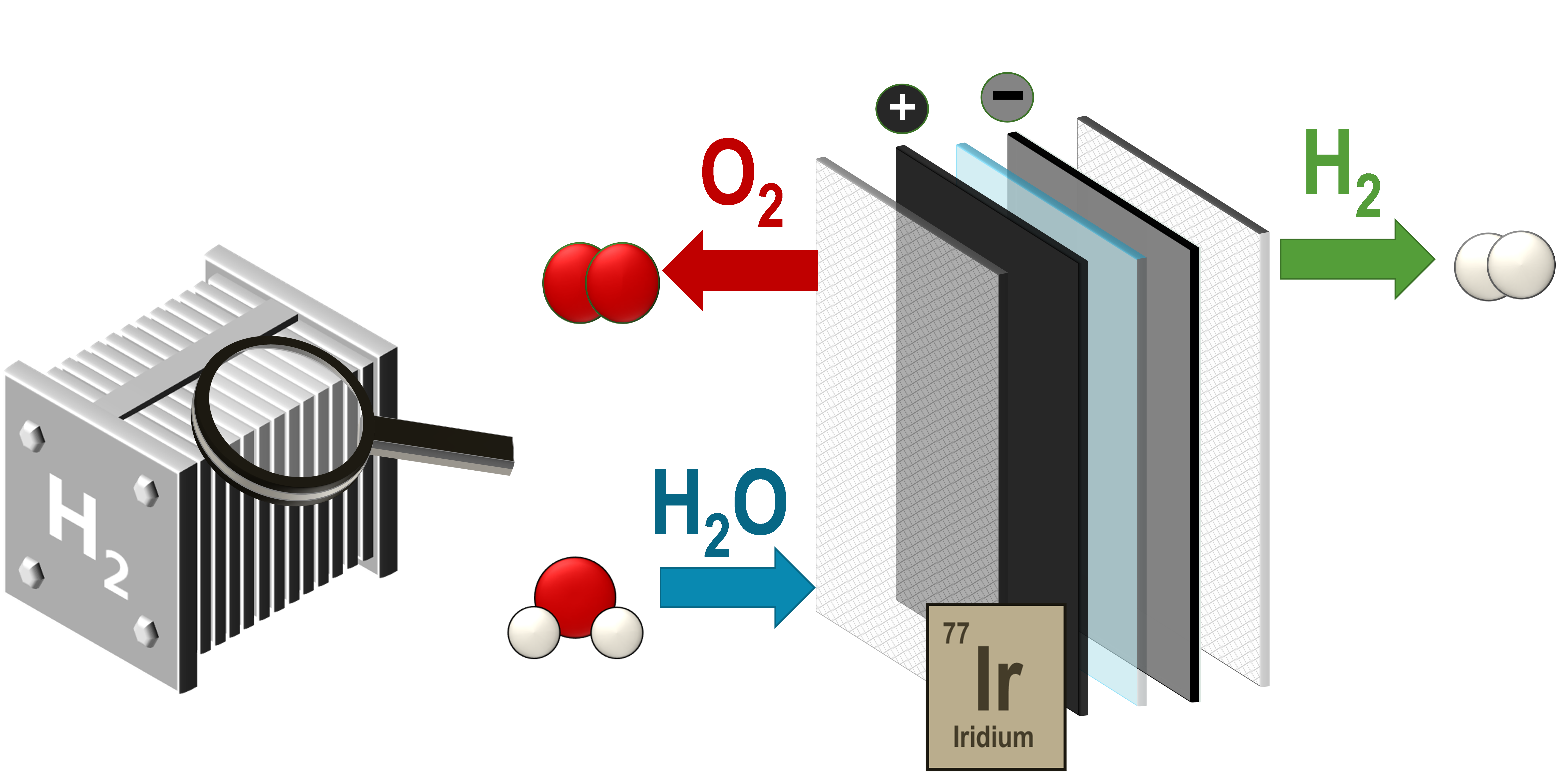
Graphic: A proton exchange membrane (PEM) electrolyzer contains a stack of membrane electrode assemblies. In each unit there is a positive and negative electrode (anode and cathode), which is separated by the polymer membrane. At the anode, water is converted into oxygen. The protons (H+) migrate through the membrane, and hydrogen is then produced at the cathode.
The most complex step in this process, which also requires the most electricity, is the development of oxygen. A catalyst of iridium oxide is used to speed up this process.
Facts: Iridium is the key
The actual splitting of the water takes place in a chamber, usually referred to as a cell, which is divided into two parts, separated by a membrane. Electricity is applied to the water in the cell so that oxygen is released in one half and hydrogen in the other.
In order to be able to split water into oxygen and hydrogen, a catalyst is needed that stabilizes the process and reduces the need for electric current as much as possible.
Today, iridium oxide is considered to be the best catalyst. However, since iridium is a rare metal, only about seven tonnes of it is produced per year worldwide. In comparison, it will take about 2.5 tonnes of iridium in Denmark alone to achieve our strategy in this area until 2030, known as the Power-to-X strategy.
Grant recipient
Rebecca Pittkowski
University of Copenhagen
Project
Stability of water electrolysis catalysts – StabilECat
Amount
6.173.070 DKK
